|
|
|
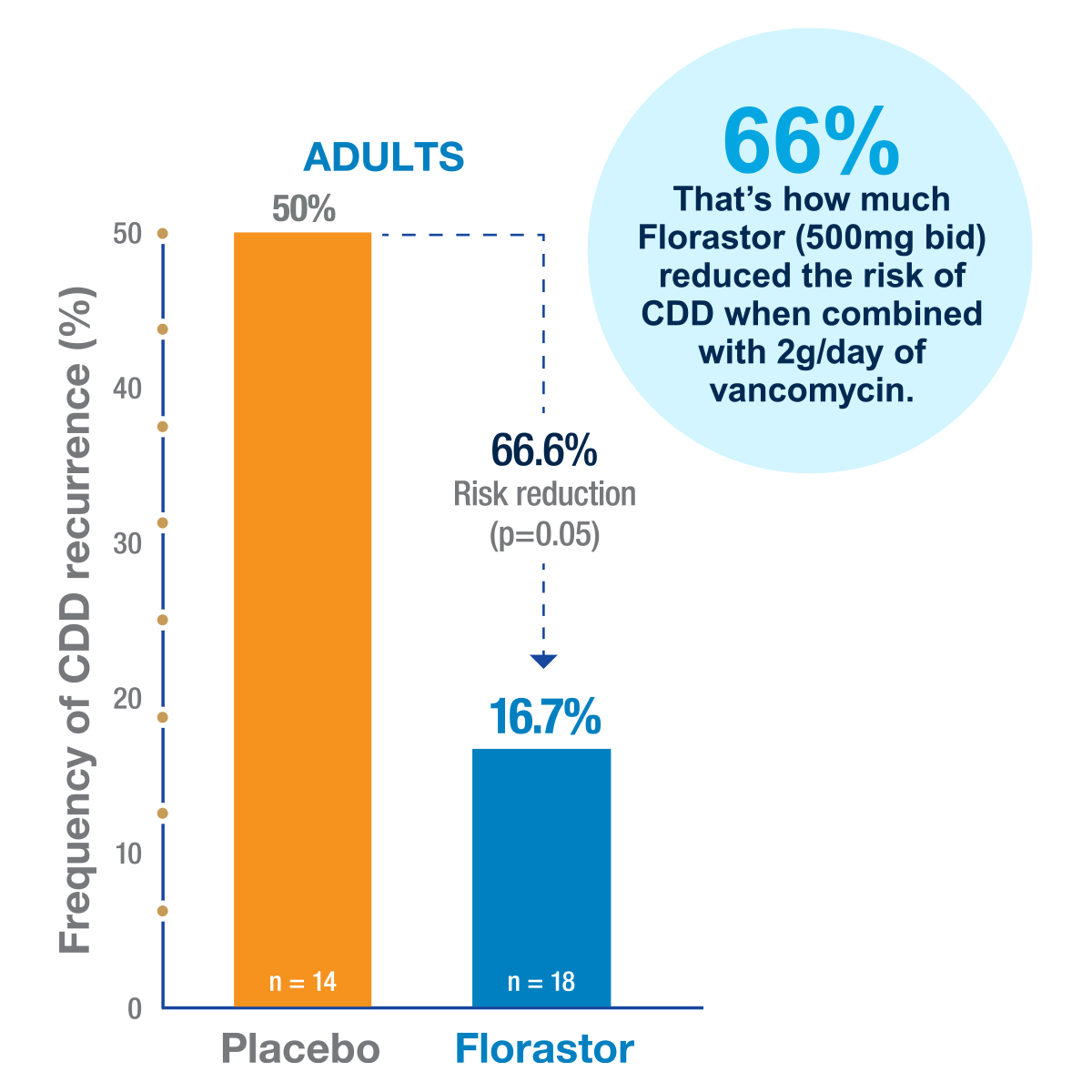
In a double-blind, placebo-controlled trial, Florastor ® reduced the frequency of recurrence of C. difficile-associated disease (CDD)1
|
|
|
REQUEST A REPRESENTATIVE
|
Learn how Florastor may
benefit patients at your hospital
or long-term care facility
|
1. Surawicz CM, et al. 2000. The search for a better treatment for recurrent Clostridium difficile disease: use of high dose vancomycin combined with S. boulardii. Clinical Infectious Diseases. 2000;31(4):1012-1017. doi:10.1086/318130
Warning:
Do not use Florastor (Saccharomyces boulardii CNCM I-745) probiotics in the acute care setting, especially in those individuals with open arterial and venous access required (such as Vascath, Permcath, or AV fistula). Do not use for any individual with a central line or port or in the surroundings of any patient with a central line or port. Central lines include short- and long-term central venous catheters (CVCs) and peripherally inserted central catheters (PICCs). Do not use Florastor for pre- or post- organ or bone marrow transplant patients and other patients considered severely immunocompromised or critically ill. Do not use Florastor probiotics if allergic to any components (especially yeast). Note: Florastor contains lactose that is sourced from milk.
|
|
|
|
|
Follow RXinsider on Social Media
|
|
|
|
|